The concept of ORP, or Oxidation Reduction Potential, is a mystery to many. It is by any measure difficult to explain and even more difficult to understand.
Often, ORP is confused as a direct measurement of the water’s sanitizer residual. It is not. It indicates the combined oxidizing power of all chemical species in the water. The sanitizer is not the only oxidizing chemical in the water: Water’s overall oxidizing power, while dominated by the sanitizer, is dictated by the oxidizing contribution from other chemical species as well.
To complicate matters more, some chemical species in the water are not oxidizers — in fact, they are classified as reducing agents, meaning they reduce the oxidizing power of the water. ORP is ultimately determined by this tug of war between oxidizing and reducing species.
For this reason, ORP is not a direct measure of sanitizer concentration but a net effect of all chemical species present.
The chemical species in a typical pool can be numerous. Consider those present in source water from a well or municipality; then look at chemicals intentionally added by pool owners and unintentionally through bathers, rainwater, runoff water, birds and other living things. There are hundreds or even thousands of inorganic and organic chemical species in a pool.
Can ORP be predicted from fundamental principles?
Every single chemical species in water contributes to its ORP value. As mentioned, there are chemicals that oxidize and those that reduce. The impact of each on ORP can be calculated if every species and its concentration are known.
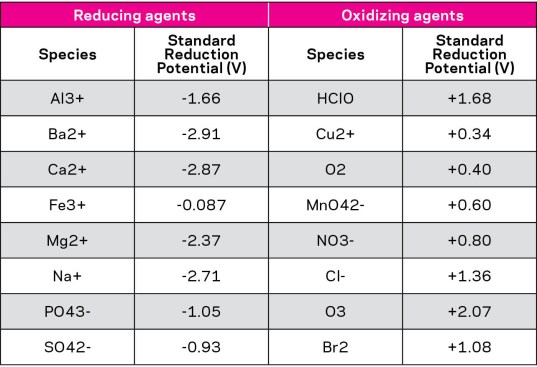
Table data source: CRC Handbook
Table 1: Electrochemical series of various chemical species relevant to swimming pool water.
This calculation requires the use of the electrochemical series (see Table 1), which provides the relative oxidizing or reducing power of various chemical species. Let’s take one simple example. Looking at the electrochemical series, we see that the addition of salt (sodium chloride, or NaCl) results in 1 atom of sodium ion (Na+) for every atom of chloride (Cl-) ion. We see that sodium is reducing in nature, whereas chloride is oxidizing. The relative strength of sodium to reduce is greater than that of chloride ion to oxidize (i.e. the sodium is more negative, -2.71 mV, than the chloride ion is positive, +1.36 mV). So the net effect of adding salt would be to lower the ORP. And the more salt is added, the more ORP is reduced.
Cyanuric acid also is known to dramatically affect ORP. In our studies, we have observed an approximate 6 mV decrease with each 10 parts per million of cyanuric acid. This is consistent with other published studies on the subject.
Finally, and perhaps the greatest known and most well documented impact on ORP comes from the water’s pH. Some studies have noted an approximate 70-80 mV decrease for each unit increase in pH. For example, the ORP will read 70-80 mV lower as pH increases by 1 full unit.
With that said, is it possible to derive the ORP for any given water solution? Theoretically yes, but it requires knowing every single chemical entity present, its concentration, and its relative oxidizing power on the electrochemical scale. Pool water has far too many unknown chemicals species present, each of whose concentrations could change daily due to makeup water, chemical addition and rain, to make such an attempt practical.
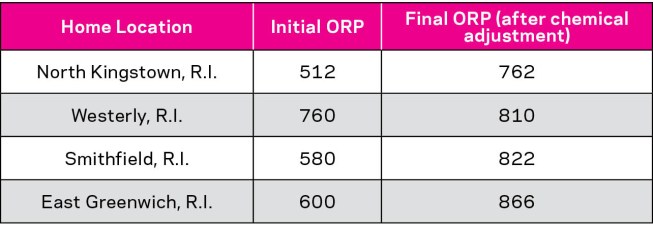
Table: Ray Denkewicz
Table 2: Four municipal tap water samples adjusted to the same pH (7.5), same chlorine (3 ppm), same total hardness (250 ppm), same total alkalinity (70 ppm) and same cyanuric acid (0 ppm) levels
To illustrate the point, water samples were taken from four different homes in the greater Rhode Island area. Each sample was adjusted for the same pH, total alkalinity, total hardness and chlorine levels. Despite consistent chemical makeup, the ORP values were vastly different (Table 2).
The differences occur because the water samples contain other chemical species native to their specific source. If one views the adjusted water samples as pool water, it is clear that the same 3ppm of free chlorine could have resulted in an ORP ranging anywhere from 762 to 866 mV.
For this reason, it is not possible to provide consumers with a specific ORP recommendation to achieve a given chlorine level. Rather, the ORP values that provides the desired free chlorine range of 1-3 ppm are site specific and must be found by trial and error by each customer.
ORP in commercial Pools
The World Health Organization recommends ORP values of 650 mV or greater for microbial-safe water. Several local health codes in the U.S. include those parameters. As a result, many commercial pool operators use ORP.
However, they also are well-versed on its nuances in real-world settings. Professionally trained operators carefully monitor, test, adjust and record the pool water quality at least daily, making ORP control more common and acceptable.
Many of these health codes naturally specify limits on the minimum and maximum free chlorine allowed during swimming activities. But in some situations, the ORP and chlorine limits are at odds with each other. Hypothetically, one could imagine a scenario where the ORP is below the requisite 650 mV ORP minimum, but free chlorine is at its maximum. In such a case, raising the ORP by adding more sanitizer would cause the sanitizer level to go above the maximum amount allowed.
This is especially possible in pools with cyanuric acid and/or salt, because the addition of these chemicals lowers the ORP even at a constant free chlorine level.
Such scenarios can be avoided if health regulators recognize that ORP values and sanitizer levels are not mutually exclusive but rather interrelated.
ORP in residential pools
ORP’s inexact relationship with free chlorine makes it a less compelling proposition for homeowners.
Pool owners are taught by the trade to maintain free chlorine levels within a specific range, so they expect a known ORP value that correlates with that chlorine level. While that is true for a given pool, it must be found by trial and error.
To utilize ORP properly, a pool owner must first balance the pool water and raise the chlorine to the desired level. The ORP of that “perfect state” can then be used as the ORP set-point. But the continuous introduction and departure of chemicals to and from the water can result in the need to change the target ORP set-point periodically. So it must be rechecked from time to time. With such variation from pool to pool, it is self-evident why the pool trade struggles with the acceptance of ORP as a mainstay method for the chemical management of residential pools.
The future of pool water chemistry management
In commercial pool operation, the use of ORP will remain for the foreseeable future as the standard sanitizer monitoring method. This is true because such language is embodied in many public health codes, the pool trade is reasonably skilled at employing ORP, and a vast majority of scientific findings around the globe support the inextricable link between ORP and disinfection performance.
In residential pool operation, however, two factors likely will keep chlorine as the preferred pool water parameter to monitor and control. First, there’s the widespread belief among consumers that chlorine is the key to safe and clean pool water. Second, there is a tremendous barrier to educate the general population on the complex behavior of ORP.
Such focus around chlorine, and not ORP, as the variable to monitor in the residential pool space will be further hardened as developments continue to unfold in the areas of app-based water chemistry test strips along with less expensive sensors for free chlorine measurement.
Ray Denkewicz is managing director, international division, and Bob Jakob is a chemical engineering technician for Hayward Pool Products in Elizabeth, N.J.
References
1. Electrochemical Series, CRC Handbook of Chemistry and Physics, 96th Edition, William M. Haynes (Editor) 2015
2. Falk, Richard A., The Chlorine/Cyanuric Acid Relationship and Implications for Nitrogen Trichloride
3. Fundamentals of ORP Measurement:Theory, Rosemount Analytical Application Data Sheet, Emerson Process Management, May 2008
4. Lachocki, Thomas M., The Impact of Pool and Spa Water, ORP, and Amperometric Controller Probes
5. Steininger, Jacques M., and Catherine Pareja, ORP Sensor Response in Chlorinated Water, NSPI Water Chemistry Symposium Series, Volume 1 (Denkewicz and Hales, Editors), 1996